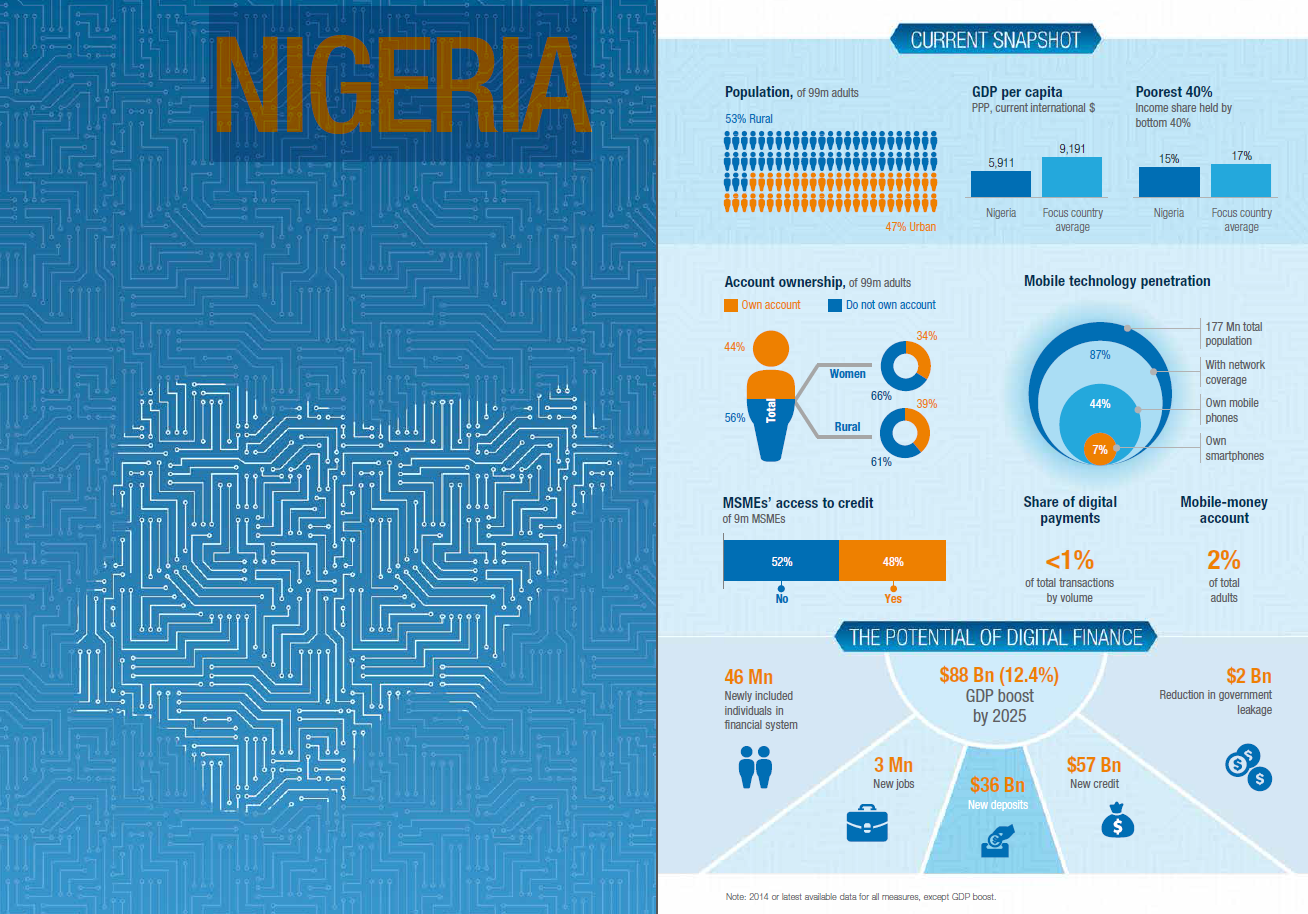
COVID-19 vaccines being imported into the country will undergo a quick clinical trial before it is administered to citizens, the Nigerian Medical Association (NMA) said on Monday.
According to the NMA President, Professor Innocent Ujah, reactions to vaccines differ among different races.
“If the vaccines come to Nigeria, we need to quickly do our own evaluation of that vaccine,” he said, during an appearance on Channels Television’s Sunrise Daily. “I cannot say with 100 percent certainty that in the process of developing this vaccine, in the clinical trials, whether any African countries were involved. I’m not too sure, but it’s possible.
READ ALSO: EndSARS: 14 Insurance Firms Settle ₦9.7 billion Claims
“Then because of the biology, because of the environment, because of our genetic composition, we need to do our own clinical trials very quickly before it can be used on Nigerians. While we do not question the efficacy and safety, the responses vary from place to place.
“Recall that when we were using chloroquine, the southern part was not responding to chloroquine, some parts of the north were. But in totality, it was thought that chloroquine was no longer effective and we changed.”