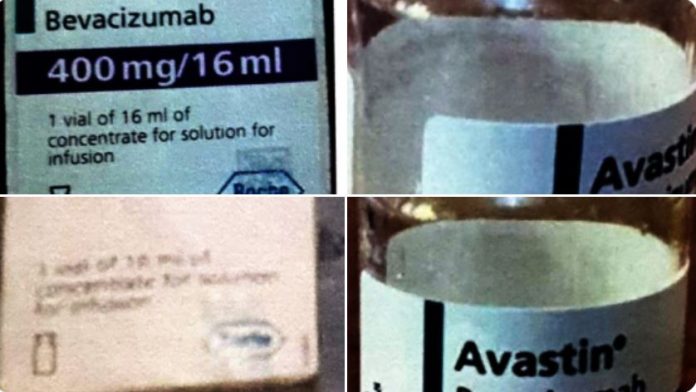
The National Agency for Food and Drug Administration and Control (NAFDAC) raises an alarm over the circulation of counterfeit Avastin 400mg/16ml, a cancer treatment drug, in Kano and Abuja.
NAFDAC identifies the affected batches as H0223B08 and H4239A70, warning that they pose serious health risks to cancer patients. These batches were flagged following reports from pharmacists and investigations confirming the products are fake.
NAFDAC provides the following details:
- Batch H0223B08: Originally manufactured by Roche and distributed to Vietnam in July 2020. This genuine batch expired in July 2022, but counterfeit versions have surfaced in Nigeria.
- Batch H4239A70: Not recognized in Roche’s database, confirming it as a falsified product.
Avastin is produced by Roche Diagnostics GmbH, Mannheim, Germany, and is used to treat various cancers, including colon, rectum, lung, kidney, brain, ovarian, and cervical cancers.
NAFDAC warns that counterfeit drugs may contain harmful or ineffective ingredients. These products can lead to poisoning, treatment failure, or reduced drug effectiveness, endangering patients’ health.
The agency urges all stakeholders, including importers, distributors, retailers, and consumers, to exercise caution and ensure that medical products are purchased only from licensed suppliers. It advises:
- Discontinuing the use or sale of the counterfeit batches.
- Submitting any suspicious stock to the nearest NAFDAC office.
- Seeking immediate medical advice if adverse reactions occur after using the counterfeit drug.
NAFDAC encourages the public and healthcare professionals to report any suspected counterfeit medicines or adverse reactions through the following channels:
- Toll-free line: 0800-162-3322
- Email: sf.alert@nafdac.gov.ng or pharmacovigilance@nafdac.gov.ng
- Online: Via the Med-Safety app or the reporting platform on NAFDAC’s website at www.nafdac.gov.ng
NAFDAC remains committed to safeguarding public health by preventing the distribution of substandard and falsified medicines across the country.