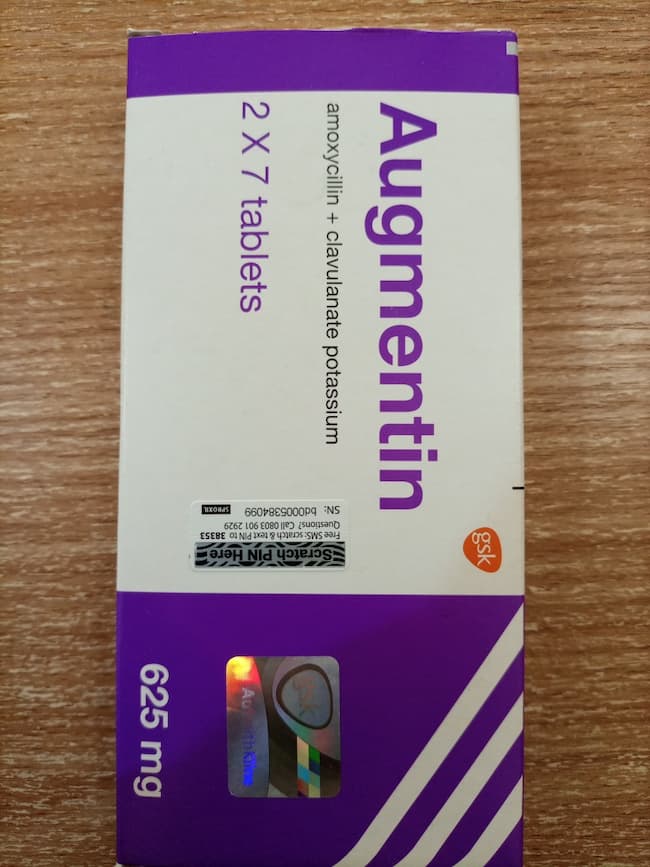
The National Agency for Food and Drug Administration and Control (NAFDAC) has warned Nigerians about the presence of counterfeit Augmentin 625mg tablets in the country.
Augumentin is an antibiotic that is used to treat various types of bacterial infections.
The agency said in a statement on Monday that the forged antibiotic has the batch number 562626, a manufacturing date of April 2021, and an expiry date of April 2024.
It went on to say that the medication has the NAFDAC registration number 04-1928 but did not meet labeling requirements.’
“No inscription “manufactured by” is written on the label, only the address.
“Manufacturing and Expiry dates do not meet the acceptable format. No MAS scratch number for verification. The logo “gsk” is not properly positioned as on the original,” the agency said.
According to NAFDAC, this indicates that the product has been falsified or counterfeited.
The agency also stated that it has notified all of its branches across the country to conduct surveillance and clean up the counterfeit Augmentin tablets.
“Please note that the genuine Augmentin 625mg has legible product labeling information including date markings – expiration and manufactured dates, batch number and NAFDAC registration number,” the statement reads.
“NAFDAC’s advice to wholesalers, distributors and pharmacies is that medicines should be obtained from authorised/licensed suppliers, increased vigilance is hereby encouraged within the supply chain to avoid infiltration of the falsified product. The products’ authenticity, physical condition and labels should be carefully checked.
“NAFDAC implores healthcare providers to ensure vigilance to prevent the administration of the falsified products on unsuspecting patients. Members of the public in possession of the above suspected counterfeit product are advised to discontinue sale or use and submit stock to the nearest NAFDAC office.
“Healthcare providers and the public should notify the nearest NAFDAC office of any information concerning the distribution, sale, and use of the falsified version of the Augmentin product.”