As countries around the world race to contain the spread of the coronavirus, securing deals with big pharmaceutical companies to produce vaccines that will be distributed to their citizens, many, especially on the African continent wonder if there is any agenda for Africa.
Coronavirus cases on the continent continue to see an uptick, with Nigeria recording over 104, 000 cases and close to 2,000 fatalities, with the citizens waiting until the promised vaccines are delivered.
In December 2020, health minister Osagie Ehanire promised that Nigerians would start to receive vaccine shots in January 2021, as Nigeria was working with the COVAX programme that is supported by the World Health Organisation.
Ehanire said, “We have 200 million citizens. We need to have a way to be able to get enough to be able to take care of our citizens.”
Awaiting the vaccine are millions of people unsure of what is expected as the government continues to restate the need for protection against the deadly disease that has endured for almost a year, adding that citizens should be wary of the resurging disease.
A photo making the rounds on the internet of a drug Cipremi/Remdesivir has piqued the interest of many, as it was stated on the package that it was not to be distributed in the United States, Canada, or the EU.
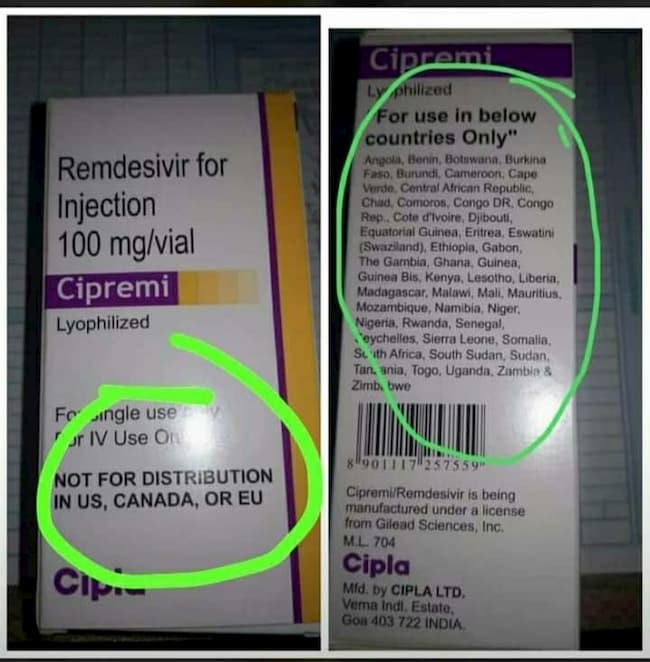
The makers, an Indian pharma CIPLA Limited, with a licence from Gilead Sciences, a US pharma, and makers of the acclaimed remdesivir, stated that the drug was to be used only in 47 countries, all African, Nigeria included.
Last year, the European Union and the US had inked a deal with Gilead Sciences, the maker of remdesivir, in the hopes that the drug would reduce the mortality rate of COVID-19 patients or ultimately quash the disease, but a different story developed altogether.
In the weeks that followed the approval for use, the World Health Organisation conducted a Solidarity trial that showed that the drug did not slow down the rate at which patients recovered, neither did it reduce mortality rates.
The organisation stated in a release, “WHO has issued a conditional recommendation against the use of remdesivir in hospitalized patients, regardless of disease severity, as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
What baffles many is that the drug has filtered into the Nigerian market despite being adjudged ineffective and the role of the drug administration and other government agencies in its entry onto the shelves of some pharmacies in the country.
BizWatch understands, from conversations with experts, that the drugs may have been products of mass production meant for Third World nations that cannot afford the higher-priced drugs available in more developed nations like the US and the EU.
Two experts are of the opinion that the National Agency for Food and Drug Administration and Control would not approve such a drug if its efficacy was in doubt.
READ ALSO: NCDC Confirms 1,867 New Cases Of COVID-19
Speaking on the issue, the President of the Pharmaceutical Society of Nigeria (PSN), Mazi Sam Ohuabunwa, said drugs that carry labels that explicitly say they are meant for African countries should be handled with suspicion.
According to him, coronavirus is a global disease all over the world and the same treatment should be administered to people irrespective of their location on the globe.
He argued that the regulatory body controlling the use of drugs in Nigeria would not approve such drugs.
Ohuabunwa said, “From the way drugs are labelled internationally, you cannot label a drug and say it is only for African countries. There is something suspicious about that. NAFDAC cannot approve a drug like that. Pharmacy and medicine are global.”
He described Remdesivir as a drug that was introduced for the treatment of the Ebola virus but was found to be non-effective.
Though the drug has been recommended for the management of the viral disease, he said it had not been effective for all those it had been administered to.
The PSN President said, “The drug is an antiviral drug made and tried for use on the management for Ebola as a therapeutic cure, but it didn’t work. Some people have tried it in the United States to manage COVID-19. It worked for some but didn’t work for many. Right now, it is no longer recommended for the treatment of COVID-19.”
US President, Donald Trump, promoted Remdesivir. It is one of the few drugs we can still use, but it is not made for Africa alone.
He stated if such a drug had been subsidised as widely claimed, it would be donated to Nigeria and not sold.
Meanwhile, President, Nigeria Medical Association (NMA), Prof. Francis Faduliye, said though he had not seen the drug, he was aware of a WhatsApp message saying the Remdesivir could only be used by African countries.
He said he didn’t believe in such a conspiracy theory because the packaging of the drug could be different from those exported by the manufacturer to other countries.
He said, “I am not aware that we have any drug that is not efficacious or of a low standard that is being exported to other countries. If that happens, every country has drug regulatory agencies and control councils and looks into such drugs and confirms if they are having the same concentration of the active substance.”
He explained that some governments visit other countries where these drugs are manufactured and negotiate ways to reduce the cost of packaging.
According to him, if the manufacturer does not want such drugs to be circulated in other countries, such instructions could be written on the label.
Contributors: Kindness Udoh, Ifeoluwa Ogunfunwa, and Oputah David












